Key words: Analytical; analysis; chemical; chemistry; education; environment; environmental; instrumentation; monitoring; training; water; water quality



For advice on and training in scientific methods for environmental monitoring
Site still under construction
1 Introduction
As with conductivity pH is an indicator of water quality and some years ago the Solway and Tweed River Purification Boards constructed the index shown in figure 1.
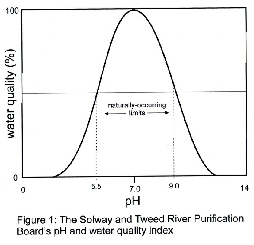 Water quality is considered high over the pH range 6.5 to 8.0 since these values
have no directly harmful effects on aquatic biota. Natural waters can have pH values
ranging from 5.5 to 9.0 but if values are encountered outside this range then they
are probably due either to unnatural discharges or to severe algal blooms.
Water quality is considered high over the pH range 6.5 to 8.0 since these values
have no directly harmful effects on aquatic biota. Natural waters can have pH values
ranging from 5.5 to 9.0 but if values are encountered outside this range then they
are probably due either to unnatural discharges or to severe algal blooms.
Metals tend to be more soluble in water of low pH and they exist as simple hydrated ions eg Mg2+(aq), Ca2+(aq), etc. Consequently, acid deposition on terrestrial environments will solubilise nutrients such as magnesium, calcium and iron causing them to be leached out into groundwater, rivers and lakes. The presence of these nutrients can stimulate the growth of algae in lakes and reservoirs, so encouraging eutrophication. In addition to the nutrients toxic metals, also, can be mobilised and leached from soil into waterways. It is in this way that aluminium enters rivers and lakes which, if they are already on the acid side of neutral, will have their pH values reduced even further by the reaction:
Al3+(aq) + H2O ® [AlOH]2+(aq) + H+(aq)
What is more, the aluminium itself is toxic to fish, precipitating out onto their gills as aluminium hydroxide and interfering with gaseous exchange across the membranes.
2 The pH of Aquatic Systems
When examining pH data for water bodies it is important to know how that data was acquired. There are two ways of measuring the pH of water samples; one gives the field pH the other gives the equilibrium pH and the two values can be very different for the same water sample, as the following graph shows.
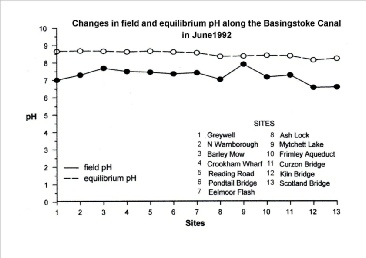 It can be seen that the field pH values vary widely, ranging from 6.58 at Kiln Bridge
to 7.91 at the Mytchett Lake. This represents a 21-fold difference in hydrogen ion
concentration. The equilibrium pH values, however, are not only significantly higher
they are less variable also. They range from 8.16 at Kiln Bridge to 8.69 at the North
Warnborough Lift Bridge; a roughly threefold difference in hydrogen ion concentration.
We will now consider the two parameters in greater detail.
It can be seen that the field pH values vary widely, ranging from 6.58 at Kiln Bridge
to 7.91 at the Mytchett Lake. This represents a 21-fold difference in hydrogen ion
concentration. The equilibrium pH values, however, are not only significantly higher
they are less variable also. They range from 8.16 at Kiln Bridge to 8.69 at the North
Warnborough Lift Bridge; a roughly threefold difference in hydrogen ion concentration.
We will now consider the two parameters in greater detail.
(a) Field pH
This is the pH of a water sample at any given moment. Thus it is the value obtained when the measurement is made on site using a pH meter. The value can vary quite considerably being dependent on the activities of living organisms, especially green aquatic plants. For example, in lime-rich waters photosynthesis causes carbonate ions to be precipitated out of solution as calcium carbonate:
Ca2+(aq) + CO32-(aq) ® CaCO3(s)
Since the carbonate ion is a base and causes water to be alkaline, its removal causes the pH to drop. It is for this reason that the pH at Greywell, where the canal emerges from chalk downland, is lower than the pH at Eelmoor Flash, which is located in acid heathland.
(b) Equilibrium pH
This is the value obtained after air has been bubbled through the water sample for twenty minutes. The water treated in this way becomes saturated with carbon dioxide which is now in equilibrium with the carbon dioxide in the air.
![]()
Because carbon dioxide has a significant effect on pH values, the point of this is to restore the dissolved carbon dioxide concentration to what it would be if there were no aquatic plants using the gas for photosynthesis. Measurements taken under these conditions give an indication of the underlying pH of the water sample; that is, the pH the water would have before colonisation by living organisms. Since the pH of aquatic systems is sensitive to dissolved carbon dioxide concentrations it is subject to regular changes because carbon dioxide concentrations themselves vary in a cyclical fashion and as the carbon dioxide concentration decreases so the pH increases and vice versa. Thus,
pH values are higher during the day and lower at night
pH values are higher in summer and lower in winter
Thus, anything which affects the rate of photosynthesis will influence pH values. Consequently, pH varies according to the temperature, cloud cover, wind velocity, etc.
3 Dissolved Material and pH
It was mentioned above that the presence of carbonate causes water bodies to be alkaline and that its removal is accompanied by a drop in pH. Of course, dissolved carbonate ions are not the only ones to affect the pH of the environment, quite a number of ionic and other materials, also, can influence the pH. Chief among these are carbonate, CO32-, and hydrogen carbonate, HCO3-, ions, which cause soil and aquatic systems to be alkaline, and aluminium, Al3+, silica, SiO2, and humic/fulvic acids, which are responsible for acidity.
Carbonate and hydrogen carbonate ions are found in lime-rich soils and waters, primarily as the calcium salts. They interact with water to generate hydroxide ions, hence the alkaline reaction:
![]()
![]()
Aluminium ions and silica also interact with water but generate hydrogen ions; hence the acidity:
![]()
![]()
The H4SiO4 is silicic acid.
Humic acids are polymeric, phenolic materials with incorporated carboxylic acid groups, RCO2H. They are derived from the peat; hence the low pH of peat bogs, peaty soils and waters draining such areas, since:
RCO2H(aq) + H2O → RCO2-(aq) + H3O+(aq)