Key words: Analytical; analysis; chemical; chemistry; education; environment; environmental; instrumentation; monitoring; training; water; water quality



For advice on and training in scientific methods for environmental monitoring
Site still under construction
For any management strategy to be effective it must be based on a sound knowledge of the situation which is to be managed. This applies as much to environmental matters as it does to anything else. However, there is a problem as far as the environment is concerned in that we know so little about it, about its structure and about the way in which it functions. Consequently, trying to develop effective management strategies is fraught with difficulties. A vast amount of time and effort goes into the study of the environment, much of which simply serves to underline the fact that our knowledge is so sparse. And so investigations continue.
Environmental research is concerned both with uncovering the basic structure of the world around us and with the quality control of the world. Both of these aspects involve some element of environmental analysis be it biological, chemical or physical. Whatever its nature, a complete analytical procedure will comprise five basic steps:
1. Identifying the object of the analysis.
2. Sampling the site/area of interest to obtain a representative sample.
3. Choosing an appropriate analytical method.
4. Performing quantitative measurements.
5. The evaluation of the results.
Of these steps students will be most familiar with step 4, having performed a number of determinations already. Step 5 involves the use of statistics, which is covered elsewhere and so will not be considered here. This leaves steps 1 and 2 which will now be considered in greater detail. However, it should be pointed out that statistics can be involved in steps 2 and 3, since sampling and analytical strategies are influenced by the nature of any statistical tests which may be applied in step 5
2. Sampling
(a) Some General Points
Many of the problems of sampling are peculiar to the particular part of the environment being studied; that is, sampling the hydrosphere involves a set of problems which are quite different to the problems involved in sampling the lithosphere. Similarly, the problems are different to those associated with sampling the biosphere, and so on. Despite these differences, however, all the problems are related to the following factors, namely the:
(i) heterogeneity of the environment,
(ii) time factor,
(iii) storage and transport of samples.
Heterogeneity: Any site under investigation is almost certain to be heterogeneous so that any small portion will not correspond to the average composition of the whole. For example, soils vary with depth, also they vary laterally as a result of the underlying geology or the way in which they are being used. Thus, an area of greensand may give way to an area of clay, or an area of loam may be partly cultivated for arable crops while the remainder is laid down to permanent grass. Each land use will have its own characteristic effect on the nature of the soil involved. In addition, two adjacent soil types will have different profiles. Similarly, water bodies have depth and breadth within which there are gradients and discontinuities.
There are times when the material to be sampled is itself homogeneous but the system may fluctuate. An example would be the periodic discharge wastewater into a river from a factory. If the rate of diffusion or dilution of the effluent is to be investigated then it would be pointless taking samples from the river when the factory is shut down or operating at reduced capacity.
The Time Factor: The levels of many materials in the environment fluctuate in a regular fashion. For example, the pH of a river or lake varies both diurnally and seasonally as photosynthesis by aquatic vegetation waxes and wanes. The concentration of carbon dioxide in the atmosphere varies from month to month (figure 1) rising to a maximum in spring and falling to a minimum in autumn. A pattern related to the combustion of fossil fuels and to photosynthesis.
This illustrates the importance of establishing base-line data so that periodic fluctuations can be identified and the range of expected results determined.
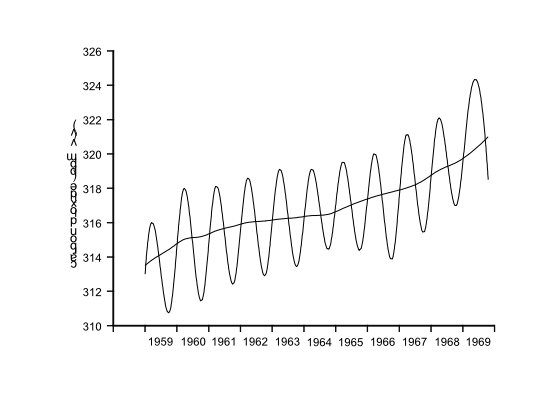
Figure 1: Variation in atmospheric carbon dioxide levels over several years
Storage and Transport of Samples: One problem of relevance, particularly to chemical investigations, is how to transport a sample from site to the laboratory without any significant changes taking place within the sample. For example, the atmospheric concentration of a pollutant such as sulphur dioxide (SO2) or ozone (O3) may have a steady state value - that is the substance is being both produced and transformed into another form at the same rate. Such is the case with sulphur dioxide which is slowly oxidised in the atmosphere to sulphur trioxide (SO3). However, when an air sample is taken that sample is often isolated from the reaction which produces the analyte whereas the mechanism for transformation is still present. Thus, by the time the sample can be analysed a significant amount of analyte may have disappeared. In such cases the analytical results are meaningless.
Other problems may arise as a result of interactions between the sample and its container. These problems are particularly serious when trace amounts are involved. Thus, when water samples are placed in glass bottles ion-exchange can take place between cationic species (positively charged ions) in solution and cations bound to the surface of the glass. It may be that the analyte is one of the ions which becomes bound to the glass. Therefore, it is removed from solution and its concentration will appear to be lower than it really is. Conversely, the analyte may be one of the accompanying anions (negatively charged ions) but the ion-exchange process releases a cation which interferes with the subsequent analysis. For example, displaced aluminium ions will interfere with analysis of fluoride since the two ions react together to form a complex which effectively removes the fluoride from solution.
Similarly, organic analytes can be adsorbed out of solution on to the surface of plastic bottles while traces of plasticiser and other organic materials can be leached out of the plastic. Also, phosphate ions are adsorbed on to the walls of plastic bottles and so water samples to be analysed for phosphate should never be taken in this type of bottle.
(b) Water Sampling
The Problems of Sampling: A body of water generally looks uniform. However, a closer examination will normally reveal that it is far from being homogeneous. Thus, lentic systems such as reservoirs and lakes can show a considerable degree of thermal stratification, as a result of which the layers of water at different temperatures may have very different chemical compositions. Concentration gradients occur as with dissolved oxygen concentrations which are high at the surface of a lake but decrease steadily with depth. Discontinuities are often present, like the summer thermocline of deeper water bodies. The amount of suspended inorganic solid material tends to increase with depth while organic solids and immiscible organic liquid pollutants of low density become more concentrated nearer the surface.
Even lotic systems are normally heterogeneous and, unless water flow is very turbulent, mixing is often incomplete and edge effects will occur near the banks and at the surface of the river/stream bed. Where a tributary or effluent from a discharge pipe enters a water course there may be a plume of unmixed material which extends for a considerable distance downstream. When sampling any water body if there is any doubt about homogeneity then samples should be taken at various points, including different depths, and under different flow conditions.
Storage of Samples: Polythene bottles are best for storing water samples before trace inorganic analysis. They are not subject to ion-exchange in the way that glass bottles are. Also, they are robust, an important consideration if samples have to be carried some distance over rough terrain. However, samples for trace organic analysis and phosphate determinations must be stored in glass bottles.
It is common practice to acidify water samples to prevent metal ions becoming adsorbed on to the walls of storage containers and to inhibit microbial activity. However, when this is done the sample should be filtered before adding the acid. This will remove any suspended matter which might be solubilised as a result of the acid conditions. When the analytes contain carbon, nitrogen or phosphorus, biocidal/biostatic agents such as toluene, chloroform, mercury(II) chloride or phenylmercuric acetate are added. These prevent the loss of analyte through uptake by micro-organisms.
(c) Soil Sampling
Heterogeneity: The chemical composition of rocks and soils can vary considerably over comparatively short distances, both horizontally and vertically. Because of the lateral variation in soil it is normal practice to collect several separate samples from points randomly distributed over the sampling area. These samples are bulked, air-dried, ground so that they pass through a 2 or 3mm sieve and then sub-sampled for analysis.
Concerning the vertical variation of soil it is often the case that, when assessing the levels of available plant nutrients, samples are taken only from the upper horizons which are influenced by ploughing. However, although sampling at this level is easy to do it does not necessarily lead to very meaningful results. From the few studies which have been made it appears that sampling to greater depths can give results which improve considerably the predictive value of available-nutrient assessments.
Another problem with soil is that its chemical properties can vary considerably with time. For example, the nitrogen content varies with the time of year, prevailing climatic conditions (especially temperature and rainfall), the use to which the land is being put and the degree of drainage.
Treatment of Soil Samples: The first step in the treatment of soil samples is usually air-drying. However, it must be remembered that soils are biologically active and drying can have a drastic effect on this activity. In particular, on air-drying the nitrogen-containing compounds tend to become oxidised so that the nitrogen is converted into nitrate. Oven-drying, on the other hand, kills the nitrifying bacteria but not the ammonifying micro-organisms. Consequently, any nitrogen tends to be converted into ammonium-nitrogen. The levels of available phosphorus, potassium, sulphur and manganese can be changed, also, by air-drying.
Nevertheless, it is essential that for most determinations the soil is air-dried because reliable sub-sampling, the next step in soil treatment, is impossible on field-moist samples. Of course, the determination of available nitrate and ammonium must be performed on field-moist soil, and in this case the samples must be stored at below 0°C. For the analysis itself the subsequent sub-sampling is done on the frozen soil. A separate determination of the soil moisture content is then made so that results can be expressed on an oven-dry basis. In this way valid comparisons can be made between different samples.
Once dried, the soil is ground down so that it passes through a 2 or 3mm sieve. If the analysis is to be performed on a 20 to 30g sub-sample little more needs to be done other than to cone and quarter the sieved soil. However, if less than 2g of sample is to be used for analysis then about 20g of the sieved sub-sample should be ground down even further so that it passes through a 100 mesh sieve. This finely-ground material is then coned and quartered.
14.3 Planning
It should be obvious that any investigation which will involve actually sampling the environment in some way has to be planned with great care. This applies whether a simple botanical survey is to be performed or some form of chemical analysis is to be undertaken. The first step in all such cases is to define, clearly and unambiguously, the object of the study. This stated aim must be very precise. For example, an aim such as “To Determine what Pollutants are Present in the River Blackwater” is far too vague. The number of potential pollutants is vast but only a certain fraction is likely to be present at any one time. The river itself is several miles long and passes through a variety of environments including urban developments, light industrial sites and agricultural land. The types of pollutant which may enter the river will vary with the location, as will the mechanism by which entry is made. Thus, industrial effluent, for the most part, enters the river at point sources while materials from agricultural land are generally transported by leaching and run-off. Some pollutants can enter the river by deposition from the atmosphere; for example, dust-borne lead.
Metals in the aquatic environment are often found to be adsorbed onto the surface of solid inorganic particles. Since these eventually settle out they carry the metals down to the river bed where they are incorporated into the sediments. Other potential pollutants may be dissolved in the water, adsorbed onto colloidal material or be complexed with organic solids.
The situation, therefore, is highly complex. Under such circumstances it would be better to look first at the activities taking place along the banks of the river, and to identify those which may generate harmful effluents. Next there is the need to identify how the effluents enter the river and what their likely composition is. This will then identify possible analytes and the forms in which they are expected to occur. It now becomes possible to define a meaningful objective for the investigation, for example, “To Determine the Levels of Chromium in the Water Column and Sediments in Relation to a Metal Plating Works Discharging into the River Blackwater.”
With a specific aim on which to focus attention can now be given to deciding which analytical method is to be used, which pre-analytical procedures to follow and, most important of all, what sampling strategy to adopt. This last will take into account the following points:
the sampling locations
the sampling sites
what number of samples to take
what size sample to take
The first two points will be decided according to the manner in which the discharge enters the river, the location of the discharge and how it disperses on entering the river. Some thought will have to be given, also, to taking samples from a site, or sites, up-river of the discharge in order to establish the background concentration of the analyte. The second two points will be decided in the light of what statistical tests are chosen to evaluate the results.
Although this has been a chemical example, the same considerations apply to biological surveys. Thus, for a vegetation survey, why is it being undertaken and what information is wanted from it? Will a line or belt transect be set up, or would it be better to devise a random block experiment? If a transect is to be used, at what distances will ,the quadrats be established? What type of quadrat will be used, a 1m2 or a point quadrat? How will the vegetation be measured? As percentage cover, frequency, or what? Given all these questions it follows that no work in the field will be effective without a great deal of careful and meticulous planning.